Abstract
The COVID-19 pandemic posed numerous logistical challenges in the way that the National Marrow Donor Program (NMDP) delivered allogeneic products to patients undergoing hematopoietic cell transplant (HCT). Prior to COVID-19, fresh bone marrow (BM) and peripheral blood stem cell (PBSC) grafts were infused into patients at the time of transplant in more than 90% of cases. Due to the pandemic, graft products could no longer be reliably delivered fresh and required cryopreservation given patient safety issues as well as logistical issues in ensuring timely delivery of a fresh product. Given these challenges, the NMDP as well as other donor registries pivoted to providing cryopreserved products. We sought to evaluate the effect of cryopreservation on the infusion of allogeneic products, analyzing all allogeneic products collected from March 17, 2020 through June 30, 2021.
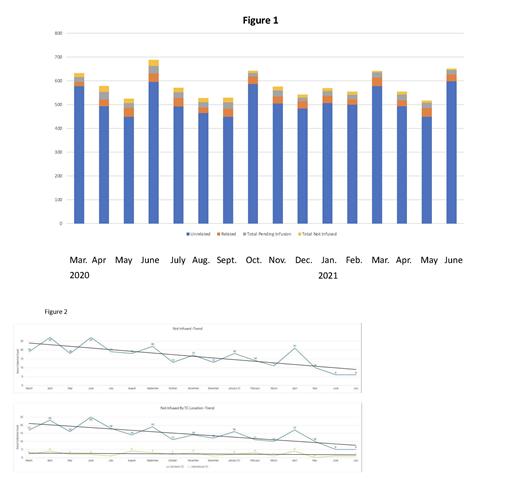
A total of 9294 products were collected from both related donors (RDs) and unrelated donors (URDs). Of these, a total of 8702 products were infused both domestically and internationally: 476 were RDs and 8226 were URDs. One main difference between fresh and cryopreserved products is that transplant centers (TCs) can wait to infuse the product depending on the status of the patient, patient's preference of when to receive HCT, and other factors. Moreover, TCs also can assess the product quality both at the time of product receipt and after cryopreservation thaw, thereby allowing the TC to determine if they will infuse a patient based on product quality. Due to this nuance for cryopreservation, 370 products are pending infusion. Over the last 18 months, only 222 products were not infused for a variety of reasons including patient death, patient choice, poor product quality (low cell counts, clumps in the product, viability or a positive culture) or unknown reasons. The number of products collected and infused on average per month was 544, while the number of products that were not infused each month averaged 14. Figure 1 shows the number of products infused, pending and not infused each month. We hypothesized that as TCs and apheresis centers became more adept at cryopreservation and infusing cryopreserved products, fewer products not infused would decrease. To determine if there was a difference in the number of products that were not infused in the first half of the pandemic compared to the latter half of the pandemic, we compared the first 5 months (March-July 2020) to the last 5 months (March-July 2021) using the Kruskal-Wallis test to compare the two timeframes. As shown in Figure 2, a significant downward trend (p<0.04) was noted in non-infused products by domestic TCs (p<0.02), but not international TCs, most likely reflecting low volume from the international TCs.
In the study cohort, we were also able to analyze the effect of COVID-19 on product infusion. Since March 2020, a total of 34 COVID-19 positive cases were noted with 33 being PCR positive and one being antibody positive. In the PCR positive cases, 16 had a donor that tested positive for COVID-19 post-donation: 12 products were infused and 4 were not infused based on the TC's preference. Thirteen (13) had a donor with COVID-19 concerns that caused the collection to be stopped and 4 were uncharacterized. In the patients that had products infused, there were no known infections of COVID-19 or deleterious effects on engraftment.
Taken together this analysis demonstrates that despite cryopreservation, a surprisingly low number of products were not infused. Moreover, patients that received products from a COVID-19 positive donor did not become infected with COVID-19 nor suffered deleterious effects. This preliminary data may help inform donors to aid them in making choices about donation as well as guide TCs and donor centers in future crises or instances when a donor's allogeneic products need to be cryopreserved
Stefanski: Novartis: Honoraria. Devine: Magenta Therapeutics: Current Employment, Research Funding; Tmunity: Current Employment, Research Funding; Sanofi: Consultancy, Research Funding; Johnsonand Johnson: Consultancy, Research Funding; Orca Bio: Consultancy, Research Funding; Be the Match: Current Employment; Vor Bio: Research Funding; Kiadis: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal